Sunday, December 13, 2015
Copper (II) Chloride Lab
Thursday and Friday in class we work on this new Copper (II) Chloride Lab. On Thursday, we measured the mass of the baby food jar first. Then my partner measured out 4g of Copper (II) Chloride and 50.0mL of water. While she was doing that, I was cleaning off our nail with steel wool so that the reaction could go faster. I took the mass of the nail and added it to the dissolved solution my partner had made.
On Friday, after it had rested for a complete 24 hours, there was some copper that had formed already. We took out the nail and washed it off into the jar. The bottom half of the nail that was in the solution looked to be about half as thick as it originally was. We then drained the liquid that was in the jar, and added 25mL of HCl to it. We then drained this out and added 25mL of distilled water and drained this also. At the end of this day, we were left with copper that is drying over this weekend.
Percent Yield
This lesson has been one of the simplest lessons we have learned this year in Chemistry. After calculating the theoretical yield and the actual yield, all there is to do is plug it into the formula. In the practice problems we were given, the problems themselves already gave us the yield, and all we had to do was use algebra to solve for either one or both of the yields. To find the theoretical yield sometimes all that must be done is the simple conversions we learned earlier.
This is how the problem is set up
These are some links that walk through the process:
http://study.com/academy/lesson/how-to-calculate-percent-yield-definition-formula-example.html
https://www.youtube.com/watch?v=Kk5kZO8ueWI
This is how the problem is set up
These are some links that walk through the process:
http://study.com/academy/lesson/how-to-calculate-percent-yield-definition-formula-example.html
https://www.youtube.com/watch?v=Kk5kZO8ueWI
Limiting Reagent and Excess Reagent
Monday in class we continued with the method that we had already learned, and applied it to solving for what are called the limiting reagent and the excess reagent. The limiting reagent whichever conversion ends up as the smaller number, and this number is the maximum amount of product that can be made. The limiting reagent is called this because the reaction will occur until this reactant runs out, and the other reactant in the reaction will be leftover in excess. The excess reagent is the larger number in these conversions, but its amount is not this number. To find the amount of excess reagent, you must take the amount of the limiting reagent and use the conversions to put it in the correct labeling for the excess. For example, if the limiting reagent was 10.5g NH3, then you would take the 10.5gNH3 and convert it to grams of Ca(OH)2, the excess reagent. Once this is converted, you will take that number and subtract it from the original amount, and finally you will know how much is left.
This link helped me the most, even though it has silly illustrations:https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/limiting-reagent-stoichiometry/a/limiting-reagents-and-percent-yield
Here are some other links that helped me with this lesson:
Website: https://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
Video: https://www.youtube.com/watch?v=qLUJdF_l8LA
These images show how some of the reactants form the products, but the red leftovers are the excess reagents we solve for.
This link helped me the most, even though it has silly illustrations:https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/limiting-reagent-stoichiometry/a/limiting-reagents-and-percent-yield
Here are some other links that helped me with this lesson:
Website: https://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
Video: https://www.youtube.com/watch?v=qLUJdF_l8LA
Thursday, December 10, 2015
Stoichiometry start
In the beginning of the Stoichiometry unit, we learned about the relationship between reactants and products in a chemical equation. We learned that by knowing just one value, we are able to calculate the values of the other reactants and products. We started by balancing a chemical equation then used the chart we were given, and personalized the problem while basing it on the chart. With this you are able to find what you want in either grams or moles, and it is very similar looking to simple conversions. Here is what the chart looks like that we used in class.:
Here are some links to help with the understanding of Stoichiometry:
Video: https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/stoichiometry-ideal/v/stoichiometry
Website: http://www.chemteam.info/Stoichiometry/Stoichiometry.html
Video: https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/stoichiometry-ideal/v/stoichiometry
Website: http://www.chemteam.info/Stoichiometry/Stoichiometry.html
Thursday, December 3, 2015
Oxidation Rules
This is a list of rules that we learned in class that we must use for classifying the oxidized and reduced substance, which are also the oxidizing agent and reducing agent.
1) Each atom in a pure element has an oxidation number of 0
2) Monatomic ions have an oxidation number equal to the charge on the ion
3) Fluorine always has an oxidation number of -1 in compounds with other elements
4) Cl, Br, and I always have an oxidation number of -1 in compounds, except when combined with oxygen or fluorine
5) The oxidation number of H is +1 and 0 is -2 in most compounds
There are exceptions:
in compounds with metals, H is -1
in peroxides, O has a charge of -1
6) The sum of the oxidation numbers for atoms in a neutral compound is 0
(no charge is shown at end)
In a polyatomic ion, the sum must be equal to the ion charge
Here are some references to go through the process of figuring them out:
Video: https://www.youtube.com/watch?v=81WdyqvLlVA
Website http://www.occc.edu/kmbailey/chem1115tutorials/oxidation_numbers.htm
1) Each atom in a pure element has an oxidation number of 0
2) Monatomic ions have an oxidation number equal to the charge on the ion
3) Fluorine always has an oxidation number of -1 in compounds with other elements
4) Cl, Br, and I always have an oxidation number of -1 in compounds, except when combined with oxygen or fluorine
5) The oxidation number of H is +1 and 0 is -2 in most compounds
There are exceptions:
in compounds with metals, H is -1
in peroxides, O has a charge of -1
6) The sum of the oxidation numbers for atoms in a neutral compound is 0
(no charge is shown at end)
In a polyatomic ion, the sum must be equal to the ion charge
| http://www.drcruzan.com/Images/Chemistry/OxidationNumbers/OxidationNumbersTable.png |
Here are some references to go through the process of figuring them out:
Video: https://www.youtube.com/watch?v=81WdyqvLlVA
Website http://www.occc.edu/kmbailey/chem1115tutorials/oxidation_numbers.htm
Metals Lab
Earlier this week we did a lab in class in which we tested the reactivity of several metals in different aqueous solutions. The lab was much more interesting to me than the others, because we could actually see the reactions happening through gas production, color changing and bubbling. We had to complete a chart on what we saw react and what we observed happening. From what we put down in our chart, we made our own reactivity series and this really helped me understand this concept. Here is a picture of one of the reactions that lasted longer, changed colors, and bubbled also:
Here's a link further explaining the reactivity series: http://www.cod.edu/people/faculty/jarman/richenda/1551_hons_materials/Activity%20series.htm
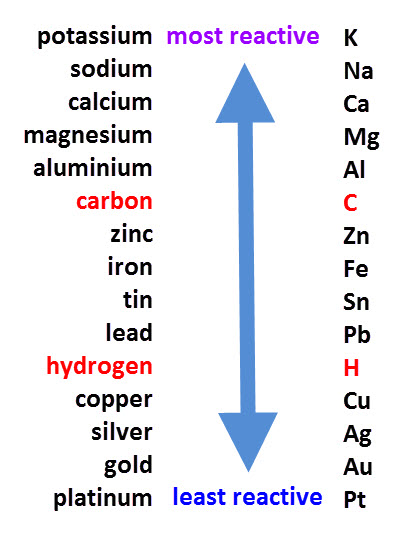
Now, this is what the standard reactivity series looks like. Those on top of the list are more reactive, and those toward the bottom are less. You use these comparisons in single replacement reactions.
Here's a link further explaining the reactivity series: http://www.cod.edu/people/faculty/jarman/richenda/1551_hons_materials/Activity%20series.htm
Now, this is what the standard reactivity series looks like. Those on top of the list are more reactive, and those toward the bottom are less. You use these comparisons in single replacement reactions.
 |
| Reactivity Series ADDED.jpg |
Sunday, November 29, 2015
Transfer of Electrons: Redox Lesson
Tuesday in class we were taught a lesson over redox reactions and the different types there are. Redox reactions consist of electrons being transferred from the metal to the nonmetal. If a species loses electrons, it is said to be oxidized, and this is considered the reducing agent. If a species gains electrons, it is said to be reduced, and this is considered the oxidizing agent. An easy way to remember this is from the acronym OIL RIG. It stands for: Oxidation is loss; reduction is gain. Here's a visual for it:
The first type of redox reaction we learned of was redox single-replacement reactions. In this reaction, the metals have changed places, the reaction is based on reactivity, and the driving force is the transfer of electrons. For this type of reaction, it it good t remember that "like attacks like". In this way, metal attacks a metal, while a nonmetal attacks a nonmetal.
The final type we talked about was combustion reactions. In this type of reaction, when a hydrocarbon reacts with water, the products are always water and carbon dioxide. Here is an example:
| http://www.ict4us.com/r.kuijt/images/en_oxidation_reduction.jpg |
The second type we learned of was synthesis. These reactions happens when two or more reactants come together and form one product. So A+B creates AB. Decomposition is the exact opposite of this, so there is one reactant breaking down to two or more products. This would then be AB creates A+B.
Here are some examples of synthesis reactions:
| http://www.biochemhelp.com/images/synthesis-reactions.jpg |
Here are some video's to further explain these concepts:
https://www.youtube.com/watch?v=RX6rh-eeflM
https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidizing-and-reducing-agents-1
https://www.youtube.com/watch?v=RX6rh-eeflM
https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidizing-and-reducing-agents-1
Acid Base Reactions Lesson
Monday during class we took our notes on reactions between acids and bases. These types of reactions will produce a water and a salt, and the water produced is the driving force of this reaction.
Here's a small example of what it would look like:
Within the acid-base reaction, there is a possibility for both strong acids and bases, as well as a weak version of both. Here are some characteristics of both.
Here's a small example of what it would look like:
| http://lrs.ed.uiuc.edu/students/mihyewon/images/HClNaOH.gif |
strong acids:
Here's a link to walk through the solving of these problems: http://science.widener.edu/svb/pset/acidbase.html
Here's a video for further explanation: https://www.youtube.com/watch?v=ANi709MYnWg
- produce H+
- protonate completely
- HCl, HBr, HI
- are the strongest if the oxygens outnumber the hydrogens by 2 or more
strong bases:
- contain an -OH- anion
- disassociate completely
- all group 1 and 2 metals plus the -OH anion are the strongest
weak acids:
- do not protonate completely
- are not on our memorized list
weak bases:
- do not disassociate completely
- are not on our memorized list
A good thing to remember is when looking at the molecular diagram, to always look for the parents. If there are more parents, this means it is weak, or if there are less, it is strong.
| http://mgh-images.s3.amazonaws.com/9780073402680/5120-4-3IRC1.png |
Here's a link to walk through the solving of these problems: http://science.widener.edu/svb/pset/acidbase.html
Here's a video for further explanation: https://www.youtube.com/watch?v=ANi709MYnWg
Tuesday, November 24, 2015
Driving Forces
This lesson was what I believe will be the first of many confusing ones. Our teacher, Mrs. Frankenberg began to teach us what driving forces are, and at least one of these must be present for two separate components to react. The various driving forces are the formation of a solid, liquid, gas, or the transfer of electrons. Driving Forces Video For Help
This lesson was focused on the formation of a solid product from aqueous reactants. This reaction is what is called a double replacement reaction where two different compounds react to create two different compounds, by switching the positive ions, or cations switching in the products. For this to occur, the products must be aqueous and ionic, and one of the products must be a solid, and in order to know which compounds are solid, we have to memorize the solubility rules.
Here are those rules:
This is the standard for this type of reaction:
This lesson was focused on the formation of a solid product from aqueous reactants. This reaction is what is called a double replacement reaction where two different compounds react to create two different compounds, by switching the positive ions, or cations switching in the products. For this to occur, the products must be aqueous and ionic, and one of the products must be a solid, and in order to know which compounds are solid, we have to memorize the solubility rules.
Here are those rules:
 |
| https://45.media.tumblr.com/tumblr_ly36p9y1NI1r0vb3to1_500.gif |
| http://study.com/cimages/multimages/16/double_displacement_reaction.png |
Monday, November 23, 2015
Chapter 7 in Class
Lately in class, we have started a new unit in class over chemical reactions. A few key things we learned to recognize if it is a chemical reaction is if: 1.) color changes 2.) a solid forms (this would be a double replacement reaction-precipitate) 3.) bubbles form 4.) heat and/or a flame is produced, or heat is absorbed. Some of the other basics we learned is within the reaction, everything to the left of the arrow is called reactants, while everything to the right is called products. Next, there are four main labelings we have been doing, these being gas (g), liquid (l), solid (s), and aqueous (aq), and these all play a part in how you go about breaking down and figuring out equations. Next, we learned that subscripts tell the number of atoms of each element in a molecule, and coefficients tell the number of molecules, and these are both clear when combining elements and balancing. This balancing I mentioned is also something we learned; it is making both sides of the reaction equal or balanced in the number of elements that are present on each side.
Here is a helpful video I found that reinforces all these basics: https://www.youtube.com/watch?v=sROBNKKJwfY
Here is a helpful video I found that reinforces all these basics: https://www.youtube.com/watch?v=sROBNKKJwfY
Solubility Lab
Friday in class we did a lab called the Solubility lab, and of course we had to pass a pre-lab to get in first. At first I did not get in because I did not properly finish the problem, but we were shown what we did wrong, and as long as we promised to never do it again, we got into the lab. The night before the lab, we were given a table where there were around 45 different double replacement or precipitate reactions. To prepare for the lab, we predicted the outcomes of the reactions, to whether it would form an aqueous product or a solid. Aqueous is a term that we learned that means it can be dissolved in water. It was very crucial in knowing the solubility rules of the products, here is a link listing all those we needed to remember:http://www.softschools.com/quizzes/chemistry/solubility_rules/quiz1333.html
Here is a picture of the chart of all the different combinations we had to predict.
Then Friday during class, we actually mixed the chemicals, and we were able to actually see in the dish if they were solid. Majority of what we predicted was right, and only a few were off, but it was fascinating to have a real- life example of what is normally seen as boring balancing and figuring out products. Here is what some of our dishes looked like after mixing:
Here is a picture of the chart of all the different combinations we had to predict.
Then Friday during class, we actually mixed the chemicals, and we were able to actually see in the dish if they were solid. Majority of what we predicted was right, and only a few were off, but it was fascinating to have a real- life example of what is normally seen as boring balancing and figuring out products. Here is what some of our dishes looked like after mixing:
Monday, November 16, 2015
Unit test Reflection
Today in class we took our exam over the entire chemical composition unit. There were 36 questions on the quiz, and although the material wasn't necessarily hard with all the practice I had done, it was still difficult to finish in time.The unit was mainly surrounded around moles, with conversions and using them in problems. The test included things such as empirical and molecular formulas with complex problems, mole road map conversions, molar mass, where you add up the periodic table masses, percent composition problems, and hydrated compounds. I did not do as well on the quiz, so here are some links that I used to help prepare myself:
Hydrated Compound Practice Problems: http://www.chemteam.info/Mole/Determine-formula-of-hydrate.html
Empirical formula help:http://www.chemteam.info/Mole/EmpiricalFormula.html
Molar mass explanation and quiz: http://www.softschools.com/quizzes/chemistry/molar_mass/quiz1120.html
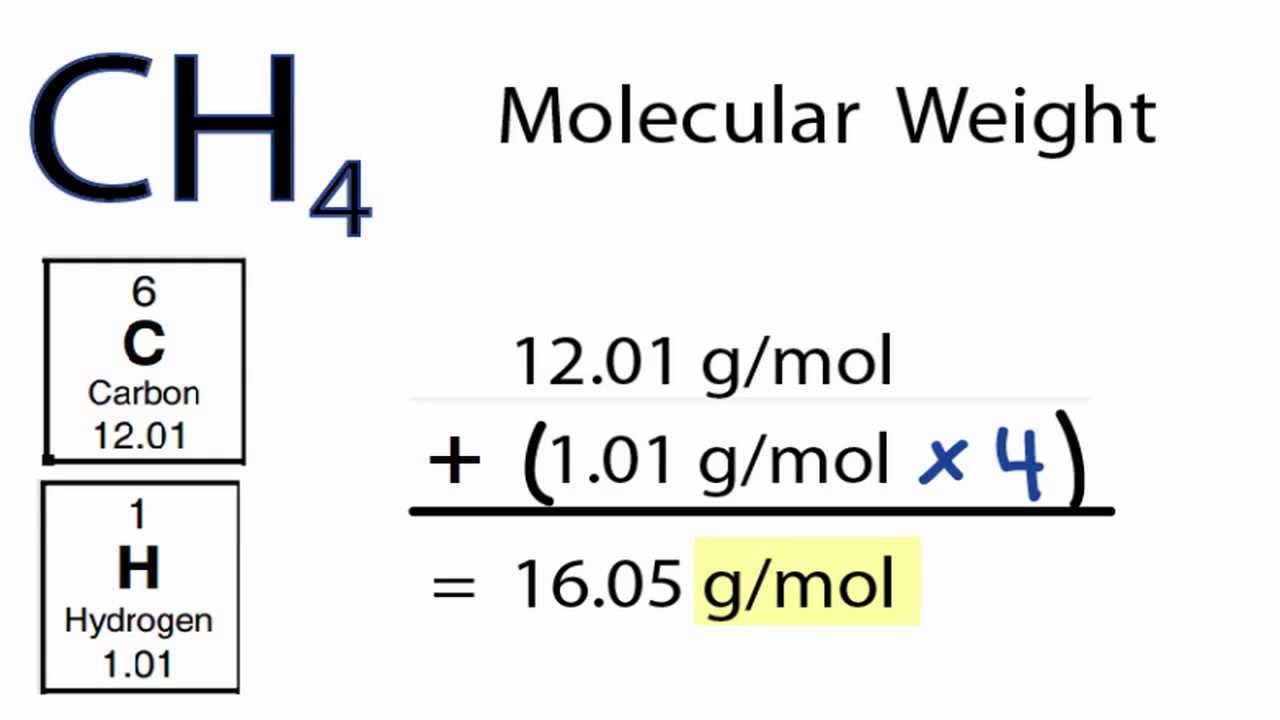
Molar mass sample:
Hydrated Compound Practice Problems: http://www.chemteam.info/Mole/Determine-formula-of-hydrate.html
Empirical formula help:http://www.chemteam.info/Mole/EmpiricalFormula.html
Molar mass explanation and quiz: http://www.softschools.com/quizzes/chemistry/molar_mass/quiz1120.html
Molar mass sample:
 |
| https://i.ytimg.com/vi/L4y8-x9ww_A/maxresdefault.jpg |
Thursday, November 12, 2015
Formula of a Chloride Lab
Today, we conducted a lab in class where we attempted to determine the formula of the compound zinc chloride by measuring the variety of masses when zinc reacts with hydrochloric acid. During the experiment, we had to measure the beaker, then add a pellet of zinc and remeasure the mass. Once this was done, we measured out 10mL of 3M HCl and added it to the beaker with the zinc. Once our partner group was ready, we placed our mixture on the hot plate and turned the heat up to around 7-8. We had to wait a while, and the reaction was done when there was little to none liquid left in the beaker. Once it was at this stage, we took it off the hot plate and let it cool off before recording the final mass. We used these three masses then to determine the empirical formula.
Here are some pictures from our experiment and my notecard of all our final calculations with the work!
Here are some pictures from our experiment and my notecard of all our final calculations with the work!
here is a basic video on the procedure, just on a larger scale!
Wednesday, November 11, 2015
Empirical Formulas
Today in class we learned about empirical and molecular formulas. There are a few key differences between the two including the empirical formula has the lowest whole number ratio and it cannot be reduced, where the molecular formula can have a reduction. It is also important to remember that the molecular formula can be the empirical formula. We learned how to find the empirical formula when you're given the percent composition of each element in the compound. When you are given this, you simply convert each percent first into grams (it will be the same exact number) then use the atomic number off of the periodic table to then convert it to moles. Once it has been converted into moles, then you will divide both numbers by the smaller number. If there happens to be a decimal, that decimal will be multiplied to reach a whole number. Remember to multiply the other numbers also by the whole number that got you to that whole from the decimal.
Here is an example of how these conversions are done!
Here is an example of how these conversions are done!
 |
| https://www.chem.tamu.edu/class/majors/tutorialnotefiles/emp2d.gif |
Thursday, November 5, 2015
Moles and Molar Mass
The past two days we have gotten started on our new unit. The first day we focused on what a mole is, that being a quantity of measurement and shown as 6.02x 10^23 representative particles. We have walked through the steps of converting between grams, volume, and mass, ultimately with moles in the middle of this action. Here is the chart that will show this process. You use this chart as essentially conversion factors to get to where you want. On the second day we were introduced to this process with compounds rather than just one element. The difference with that process is that the atomic mass must me multiplied by the amount there are. For example, C3H8 would have to multiple the atomic mass of carbon by 3 and hydrogen by 8.
Here is a video I found explaining the road map: http://m.youtube.com/watch?v=mBVL0PHPrhg
Tuesday, November 3, 2015
Pre-Test
Monday in class we took the entire period doing a pre-test for our upcoming unit. To be honest, I was completely lost during the entire test and I didn't know how to solve any of the problems. Most of the problems were numerical, so if I didn't know the formulas of how to figure them out, I had to guess. I did some research and found some websites to help going into this unit.
This link will help with basic understanding when just starting to dig in:
http://easyscienceforkids.com/chemical-composition/
This link goes more in depth of empirical formulas and how to do the calculations:
http://pages.towson.edu/ladon/empiric.html
Here's a picture of how working out empirical formulas will look:
This link will help with basic understanding when just starting to dig in:
http://easyscienceforkids.com/chemical-composition/
This link goes more in depth of empirical formulas and how to do the calculations:
http://pages.towson.edu/ladon/empiric.html
Here's a picture of how working out empirical formulas will look:
| http://web.tenafly.k12.nj.us/~shilfstein/emp1c.gif |
Thursday, October 29, 2015
Last Meal Project
Each unit we cover in class, there is a project that goes along with it, which we conduct ourselves outside of class. This unit the project is called the Last Meal Project. If it weren't already obvious, we are picking 3 specific foods we would want included as our last meal; the meal includes an appetizer, main course, and dessert. The three dishes I chose were Hot Crab Dip, Chili, and Pumpkin Snickerdoodles, which I find all very yummy and ideal for this last meal. The project called for us to convert all the english measurements into metric units. You can find all my work on my Last Meal Conversion Project Page.
Here is the link to the page that was extremely helpful for my conversions:
http://www.jsward.com/cooking/conversion.shtml
Here's a little sneak peak of what my conversions turned out to look like:
Here is the link to the page that was extremely helpful for my conversions:
http://www.jsward.com/cooking/conversion.shtml
Here's a little sneak peak of what my conversions turned out to look like:
I did have a lot of fun with this project, especially since I explored Pinterest a lot for these three recipes and more! We also have to bring into class one of our dishes, and I chose to bring in the Pumpkin Snickerdoodles. I had never made them myself before, and now this is one of my favorite recipes, and it will definitely be a popular treat this fall!
Monday, October 26, 2015
Matter and Measurement
Today we took a quiz in class over all the material we have learned over the past week. This includes the states of matter, properties, and separation methods covered in one of my last posts. Other material we covered later in the week was accuracy and precision, significant figures, and conversions in the metric system. The topic I found myself struggling with the most when studying for this quiz was significant figures and heterogeneous and homogeneous mixtures. I have trouble remembering which zeros are significant when they are in front of the decimal or when there is no decimal present. Another silly mistake I believe I made of the quiz is switching accuracy and precision. After looking over my notes later in the day I found that accuracy is the proximity of a measurement to the true value of a quantity while precision is the proximity of several measurements to on each other. Also, accuracy depends on the instrument you are using, while precision depends on you as an individual. Our unit test is Thursday and I will be sure to focus on these things I struggled with on the quiz.
Here are some practice worksheets I will be using in order to prepare for the unit test
http://www2.bakersfieldcollege.edu/dkimball/Chemistry%20B2A/Problem%20set%201%20significant%20figures%20Answer%20Sheet.doc
https://www.everettcc.edu/files/students/rainier-learning-center/tutoring-center/chemistry/w316-significant-figures-worksheet.pdf
http://myweb.astate.edu/mdraganj/Sigfig1.html
Here are some practice worksheets I will be using in order to prepare for the unit test
http://www2.bakersfieldcollege.edu/dkimball/Chemistry%20B2A/Problem%20set%201%20significant%20figures%20Answer%20Sheet.doc
https://www.everettcc.edu/files/students/rainier-learning-center/tutoring-center/chemistry/w316-significant-figures-worksheet.pdf
http://myweb.astate.edu/mdraganj/Sigfig1.html
Sunday, October 25, 2015
Mole Day
Friday in class, we celebrated mole day. For this, we has to sew a mole of our own that had a theme to it. My mole was cookie monster was cookie monster or "cookie molester", and along with my friends' moles, elmo and oscar the grouch, we were able to make sesame street. During class we were able to look at the moles from all the classes and some of them were very funny and their sewing and decorations looked great, while others didn't as much. My mole took me a long time to make, not because it was difficult, but because I struggled sewing when my thread knotted up all the time. Overall, I didn't think the project was too bad and it was nice to have down time in class to celebrate this day. Heres some pictures of my mole and the three moles of our theme together.
I also didn't really understand the reasoning behind why we were celebrating, so I looked it up and here is a link to what I found. http://www.moleday.org
I also found some jokes to go along with the day that some of my friends and I got a kick out of. http://www.mytowntutors.com/2014/10/mole-day-jokes-top-50-mole-day-jokes/
I also found some jokes to go along with the day that some of my friends and I got a kick out of. http://www.mytowntutors.com/2014/10/mole-day-jokes-top-50-mole-day-jokes/
Wednesday, October 21, 2015
States of Matter
Today in class we covered the chapter in our book over states of matter. Some important terms we learned were intramolecular and intermolecular. Intramolecular includes ionic, covalent and metallic, where the break changes the identity of the material. On the contrary though, intermolecular associates the neighbors and includes hydrogen bonds, and when broken, the phase changes. We also covered the physical properties and changes, as well as chemical properties and changes. Physical changes can been easily observed without the substance changing into another. The opposite of this would be the chemical side, where the substance changes and results in a completely new substance. So determine which type of reaction it is, it is easiest to analyze if it is the same substance as what you began with. Finally, at the end of class we covered filtration, distillation, and chromatography, which are techniques to separate and identify the different components within the substances. This is the part of the lesson that confused me the most, since I haven't learned it before, but I think with a small amount of research, it should me a concept that I can learn quickly.
I have even found a small video here that explains it!
https://www.youtube.com/watch?v=iX4WlKIAuYg
Here is also a chart we used in class that was very helpful in organization and figuring out problems!
I have even found a small video here that explains it!
https://www.youtube.com/watch?v=iX4WlKIAuYg
Here is also a chart we used in class that was very helpful in organization and figuring out problems!
| http://wpscms.pearsoncmg.com/wps/media/objects/3661/3749680/Aus_content_01/Fig01-05.jpg |
Thursday, October 8, 2015
Aspirin day 2
Since we did not do the experiment ourselves, all my observations are unfortunately coming from what I have heard and seen of others.
On this second day, we would have filtered the solution that was previously created on day 1 with a Buchner funnel. The funnel pulled out all of the water and other liquids within the sample, and all that was left present in the funnel were the crystals. With the final product, then a scale was used to make the measurements of mass. The three things that had to be weighed were the filter paper, starting material and the watch glass. After break we will be able to see the sample as dry crystals. I am kind of bummed we did not get to participate in this lab, especially because it carries through the rest of the year.
Here is a video I found that may help explain the basics of the experiment:
https://www.youtube.com/watch?v=Y4NMpO1xI8U
This is what the lab setup would have looked like for today, and the measurements my friend got with her experiment- that I will be referring to for the rest of this school year.
On this second day, we would have filtered the solution that was previously created on day 1 with a Buchner funnel. The funnel pulled out all of the water and other liquids within the sample, and all that was left present in the funnel were the crystals. With the final product, then a scale was used to make the measurements of mass. The three things that had to be weighed were the filter paper, starting material and the watch glass. After break we will be able to see the sample as dry crystals. I am kind of bummed we did not get to participate in this lab, especially because it carries through the rest of the year.
Here is a video I found that may help explain the basics of the experiment:
https://www.youtube.com/watch?v=Y4NMpO1xI8U
This is what the lab setup would have looked like for today, and the measurements my friend got with her experiment- that I will be referring to for the rest of this school year.
Aspirin lab day 1
Today during class we were supposed to begin the process of making Aspirin in our lab. Unfortunately, my partner and I did not pass the pre-lab quiz and therefore couldn't participate in the lab. For our pre-lab quiz, we were given a sentence directly from the procedure packet, and we had to fill in the blanks. This challenged us to know terms such as water, excess, acetic anhydride, and crystallize to fill these blanks. Because we did not conduct the experiment ourselves, I had to get the data from a friend, and now for the rest of the year I will have to refer to her sample and the calculations she gathers with the sample her and her partner made.
If we were to have conducted the lab today, we would have included the following:
1. Combining in an Erlenmeyer flask, the 5g of salicylic acid with the 7g of acetic anhydride, and then 8 drops of concentrated sulfuric acid to this mix.
2. We would have then heated these materials inside the flask by submerging it into a water bath using a hot plate. We would have left this to heat up for fifteen minutes.
3. The last step for this day would have been to wait for the mixture to cool for three minutes, then add 15mL of ice cold water and stirred to cut down o the exothermic-ness.
Here is the link to access our full procedure we were to conduct in class:
http://www.chem.latech.edu/~deddy/chem104/104Aspirin.htm
Some things we altered about the procedure were to use a hot plate instead of a bunsen burner, and to use ice cold water instead of room temperature in 4B.
This is what the setup of day 1 would have looked like:
If we were to have conducted the lab today, we would have included the following:
1. Combining in an Erlenmeyer flask, the 5g of salicylic acid with the 7g of acetic anhydride, and then 8 drops of concentrated sulfuric acid to this mix.
2. We would have then heated these materials inside the flask by submerging it into a water bath using a hot plate. We would have left this to heat up for fifteen minutes.
3. The last step for this day would have been to wait for the mixture to cool for three minutes, then add 15mL of ice cold water and stirred to cut down o the exothermic-ness.
Here is the link to access our full procedure we were to conduct in class:
http://www.chem.latech.edu/~deddy/chem104/104Aspirin.htm
Some things we altered about the procedure were to use a hot plate instead of a bunsen burner, and to use ice cold water instead of room temperature in 4B.
This is what the setup of day 1 would have looked like:
Thursday, October 1, 2015
Unit 2 Test Reflection
Today during our chemistry class, we took our unit test over atomic structure and radioactivity, which we have been covering for the past several weeks. The test covered determining the number of protons, neutrons and electrons, mass number, atomic number, calculating average atomic mass, abundance, radioactivity and the three particles included in that, fission and fusion, and the scientists and their experiments and the resulting theories. Overall, I think I was more prepared for this test than I had expected to be. I set aside a lot of time for preparation, and it appears it ended up paying off. The only questions I struggled with at first were half life, but when I went back to these questions I realized I had been overcomplicating the problems. From there I was able to easily find and check my answers for these problems. I think half lives will be a topic I will need to review before taking the next quiz or test. I am very confident in my performance today and I am excited to see what material we will be covering in the next unit!
Wednesday, September 30, 2015
At Home
As I have been learning more and more about half lives and have been preparing for our unit test, I have had a great amount of struggle in memorizing the methods. There are a few ways this can be approached, and I did not fully understand the explanation in class. I did, however, find this link that was very helpful in working out these problems. Now, I will be more prepared for our unit test that includes half life problems. Here is the link for the problems I found! http://www.chemteam.info/Radioactivity/Radioactivity-Half-Life-probs1-10.html
Tuesday, September 29, 2015
Monday and Tuesday in Class
Over the past two day in class, we have been working on an archeology lab that requires us to deal with half lives. On Monday all we got done was cutting out the 567 squares that represented atoms from the radioactive remains from deer hunter's discovered skeleton. We are trying to determine which of the missing persons match the skeleton found based on the decay of the skeletal remains. Outside of class, we were required to conduct the experiment in which we shook the box of the 567 squares and took out the squares that landed on the colored side. Each time we would count this number of squares then do the same for the squares remaining in the box. Today in class we were able to put together our graph and determine the missing person off of our decay results. I found this very interesting that a small system like this would be able to match a deceased person to their identity. Here are pictures through the process of taking out the colored squares and counting them.
Sunday, September 27, 2015
Thursday in Class
Today in class we began to learn about the radioactivity of many nuclei. We were given the three different types were alpha, beta and gamma. One very helpful concept we learned while calculating these three is that the two daughters in the equation will always add up to the parents.
Alpha is shown by 4 He
2
Beta is shown by 0 e
-1
And gamma is shown by 0Y
0
Here is a helpful chart we made in class for remembering the differences between the three types
One very helpful concept we learned while calculating these three is that the two daughters in the equation will always add up to the parents. So, this picture shows alpha radiation because there is the 4He, and you can also notice that the 2 and 86 add up to be 88 and at the same time 4 and 218 add up 2 to be 222. These concepts are much easier now that i was shown how simple it is to just add them up. We also learned how to predict the daughters when we know the parent and the type of radiation. Just take the parent function and add or subtract the numbers corresponding with the radiation. This is what it would look like and all there is to do is find the number that will add together with the decay to equal the parent. In this problem, the ? would end up being 234 Th
90
Alpha is shown by 4 He
2
Beta is shown by 0 e
-1
And gamma is shown by 0Y
0
Here is a helpful chart we made in class for remembering the differences between the three types
One very helpful concept we learned while calculating these three is that the two daughters in the equation will always add up to the parents. So, this picture shows alpha radiation because there is the 4He, and you can also notice that the 2 and 86 add up to be 88 and at the same time 4 and 218 add up 2 to be 222. These concepts are much easier now that i was shown how simple it is to just add them up. We also learned how to predict the daughters when we know the parent and the type of radiation. Just take the parent function and add or subtract the numbers corresponding with the radiation. This is what it would look like and all there is to do is find the number that will add together with the decay to equal the parent. In this problem, the ? would end up being 234 Th
90
It is also important to remember to change the element letter if you notice that there is a change in the proton count, or the bottom number!
A helpful video that breaks down writing the formulas is: https://www.khanacademy.org/science/chemistry/nuclear-chemistry/radioactive-decay/v/alpha-beta-and-gamma-decayTuesday, September 22, 2015
9/22/15 Today in Class
Since we have been discussing things such as the abundance and average atomic mass, today in class we applied what we have learned through lab called Beanium. At first, to get into the lab, we had to answer a pre-lab question in which we had to calculate the average atomic mass when we were given the mass and the percent abundance. Thankfully, the pre-lab question was much easier than I expected and I was able to participate in the lab. I thought the lab was very straightforward and relatively easy to conduct; it did not take us hardly any time to finish since all we had to do was weigh and do calculations. It was nice to be able to incorporate a lab into what we have been learning and see how the concepts are used even through beans.
Here is a link to explain and practice the formula and how to calculate average atomic mass!
http://www.chemteam.info/Mole/AverageAtomicWeight.html
Here is a link to explain and practice the formula and how to calculate average atomic mass!
http://www.chemteam.info/Mole/AverageAtomicWeight.html
Monday, September 21, 2015
9/21/15 In Class
Today during class we began reviewing and learning more about the modern concept of the atomic structure and isotopes. Some of the material, such as basic concepts about protons, neutrons, and electrons, I was already familiar with. However, it was very refreshing to review these small pieces as we transitioned into learning about isotopes. During the review questions in class, I had some difficulty understanding and completing an isotope notation, but now that I have done some extra review, I am much more confident and comfortable with the material. I had some confusion between the mass number and atomic number, but now that I have that clarified, it is much easier to comprehend these notations. In class we also learned how to calculate weighted averages, which I didn't find extremely challenging since it is based of a formula. As we practice with these in class, I am hoping I am able to complete these problems in the smallest amount of time possible and with minimal obstacles. This simple diagram shows the basics that we have been applying in class
I also found a very helpful video for the average atomic mass calculation, which I was slightly confused on. This video clarified in easy to understand steps how to complete this process.
http://pad2.whstatic.com/images/thumb/0/0f/Find-the-Number-of-Protons,-Neutrons,-and-Electrons-Step-5.jpg/670px-Find-the-Number-of-Protons,-Neutrons,-and-Electrons-Step-5.jpg
Sunday, September 20, 2015
Thursday in Class
Since we have been learning about atoms and molecules in class this week, we are also going to expand our knowledge as we apply it to astronomy. We are beginning a project that we have to create an online data base which tracks the Elements in the Stars. I have never been very interested in astronomy and it has been a topic I typically avoid, especially since it is not something that I can physically study and observe often. I am hoping this project sparks my interest a little more and I am able to learn a great amount from it. From the little research I have done, it seems that the project will associate fusion, which I believe was covered in the pre-test we took. Hopefully we learn more about this process in class and I am able to apply it easily to this project. Here is a link to the video and text that has given me some background to my project. The Elements: Forged in Stars
Thursday, September 17, 2015
During class
Now that we have started a new unit, we have started learning about new things involving the Atomic Theory and Nuclear Chemistry. Yesterday, one of the new theories we learned about was Dalton's atomic theory, the Law of Constant Composition, as well as experiments conducted by JJ Thompson and Rutherford. I found it very interesting to see who was able to come up with these theories, and the process of how we were able to obtain knowledge on atoms overall. I am looking forward to being able to concrete these theories and applying them also. I found this timeline that was extremely useful to summarize the actions of each individual, and I will be referencing this frequently until i have fully memorized the process.
https://2011modelsa.wikispaces.com/file/view/timeline.jpg/259066848/968x578/timeline.jpg
Here is a video that further explains the models and how the structure of the atom was determined!
Tuesday, September 15, 2015
9/15/15 during class
Today during class we took a pre-test on different atomic structures and radioactivity. Unfortunately, I struggled a lot and knew little to none of the material on the test. This does mean, however, that I have a lot to learn this unit. I think I will be learning much more this chapter compared to the other since I did have some of a background to it beforehand. This excites me to see how much new ground I will be covering through this next unit. I also found this diagram that could potentially help me in underlying knowledge for this upcoming unit. Although it is fairly simple, I think it could get me a good head start for the first lecture.
I have found a video that covers what I found on the test and what I believe we will be covering in class soon!
Monday, September 14, 2015
Naming binary compounds
Another topic we have been covering during in class is how to name the three types of binary compounds. At first, I struggled memorizing the process as to how to name each type, but I found this helpful flowchart to help me study! With the naming broken down into simple steps and drawn out like this, it was much easier for me to remember the process.
Now I can use this flowchart in my head when going through any binary compound problems in the future without having any problems.
Friday, September 11, 2015
Naming Acids!
Lately, we have been learning how to properly name different things in chemistry. One of the topics we have covered is how to name acids! I found this very helpful flowchart that makes it much easier to go through the steps of successfully naming these acid!
One other helpful saying that we learned in class was "no o hydro". I found this saying to easily stick in my mind and it is a very effective saying when trying to memorize naming acids!
I also was able to find a simple youtube video that went step by step to refresh my memory in the steps of naming an acid!
That video can be reached by this link:
Thursday, August 20, 2015
Introduction Page
Hi this is Holly! I am a junior at FHHS and this is my Pre-AP Chemistry blog! I am in many clubs including Student Council, Viking Leadership Council, NHS, Viking Edge, Chem Club, HOSA, and FCA. I have a strong interest in medical sciences and have also developed an interest in biomedical sciences, and I hope to develop a career through these. I also play soccer for club and school teams. In other free time I enjoy hanging out and having fun with friends. (:
Subscribe to:
Comments (Atom)





























