To start, we went through the flow chart to determine what kind of inter and intramolecular forces were within a compound.
This is the flow chart that we followed, and we went over being polar and how hydrogen bonding examples needed to make FONCLs (phone calls).
| http://schoolbag.info/chemistry/central/central.files/image1460.jpg |
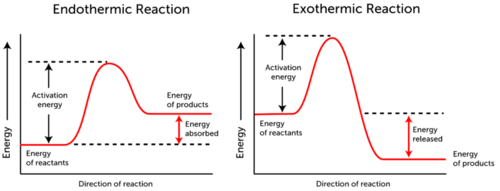
Next we looked at various curves so we could see the differences in phase changes. This is similar to what ours looked like.
 |
| https://writer.zoho.com/image.do?imgurl=43f284c1e0ba6646e2d2a98b8a52495191ec6d7cf7d22a0458f6d938d98b4eb1a7800cecd6ac3d9ca48373fdd0cecca6 |
| http://www.kentchemistry.com/images/links/matter/Phase.gif |
Here are some extra links for practice:
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch14/phase.php
http://www.sciencegeek.net/APchemistry/APtaters/PhaseDiagrams.htm
https://www.khanacademy.org/test-prep/mcat/chemical-processes/covalent-bonds/a/intramolecular-and-intermolecular-forces