Next we learned about end and exothermic processes.
Here is a picture demonstrating how endothermic take in energy and exothermic release energy
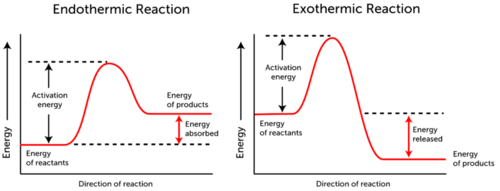 |
| https://dr282zn36sxxg.cloudfront.net/datastreams/f-d%3Adf0a2687d885c997ec852a60b09181c51b0a234ada9136e0288d4e8c%2BIMAGE_THUMB_POSTCARD%2BIMAGE_THUMB_POSTCARD.1 |
to plug into this formula, you must know that Q is heat in joules, m is mass in grams, c is specific heat in (J/g C), and T is change in temp.
This shows the process:
 |
| https://i.ytimg.com/vi/0jKHtBJNAYM/maxresdefault.jpg |
 |
| https://i.ytimg.com/vi/vQ6VIHqfVLc/hqdefault.jpg |
Here are some more links for practice and understanding of this:
No comments:
Post a Comment