The second day in lessons, we added to our gas laws and learned Charle's Law. This states that temperature and volume vary directly with each other, using the same formula, and this is at constant pressure. It is important to remember that this also must be done in Kelvin, so to convert from celsius to Kelvin, you have to add 273.15 to the original C.
This is what the formula is directly for this, and what it would look like:
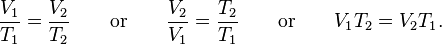 |
| https://upload.wikimedia.org/math/9/6/8/9682f75ffd644c1e723156ad5919c8a6.png |
 |
| http://wps.prenhall.com/wps/media/objects/4678/4790892/images/aabjvhoa.jpg |
The graph of this relationship would also look like this:
 |
| http://chemwiki.ucdavis.edu/@api/deki/files/8688/=CharlesLaw_(2).jpg?revision=1 |
Here are more links for practice:
http://www.chemteam.info/GasLaw/WS-Charles.html
http://science.widener.edu/svb/tutorial/charleslawcsn7.html
Your summary was well written and gave a good description of the Law. I liked the pictures you added on to the post also, they helped visually show the concept and law more!
ReplyDelete