Today in class we took our exam over the entire chemical composition unit. There were 36 questions on the quiz, and although the material wasn't necessarily hard with all the practice I had done, it was still difficult to finish in time.The unit was mainly surrounded around moles, with conversions and using them in problems. The test included things such as empirical and molecular formulas with complex problems, mole road map conversions, molar mass, where you add up the periodic table masses, percent composition problems, and hydrated compounds. I did not do as well on the quiz, so here are some links that I used to help prepare myself:
Hydrated Compound Practice Problems:
http://www.chemteam.info/Mole/Determine-formula-of-hydrate.html
Empirical formula help:
http://www.chemteam.info/Mole/EmpiricalFormula.html
Molar mass explanation and quiz:
http://www.softschools.com/quizzes/chemistry/molar_mass/quiz1120.html
Molar mass sample:
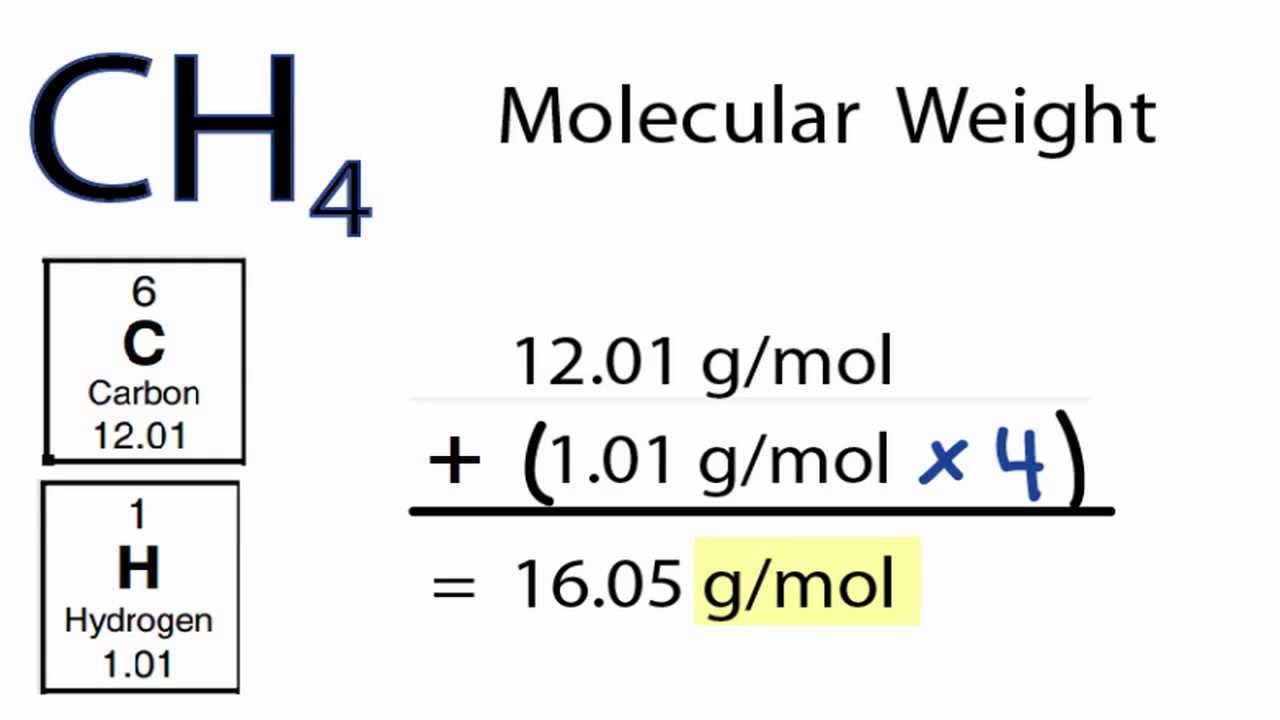 |
| https://i.ytimg.com/vi/L4y8-x9ww_A/maxresdefault.jpg |

These links are really helpful, and I wish I would've used these before the test to study! I'm not sure how I feel about this test, but it's great that you were so well prepared considering how hard it was!
ReplyDeleteThis blog post is great and it includes so much information. These topics were a few of the main things that I struggled with throughout the unit, and these links helped clear some things up for me. I wish I would have seen these before the unit test, because I am sure that they would have helped. Maybe next time include more of your own explanation, rather than only links. Other than that, this post was great, thank you!!
ReplyDeleteThese links are really helpful and I'm glad you have a specific example displayed on your post.
ReplyDeleteI agree with you that I did not have enough time during the test. These links are great and I wish they were available to me before the test because I am sure they would have helped! Thanks for sharing!
ReplyDeleteI too, like Lauren, struggled to finish the test on time due to the amount of work required by each problem. I really found the links you posted to be helpful in my studies. I am grateful that you provided an easy-to-understand example, yet the example was very descriptive.
ReplyDelete