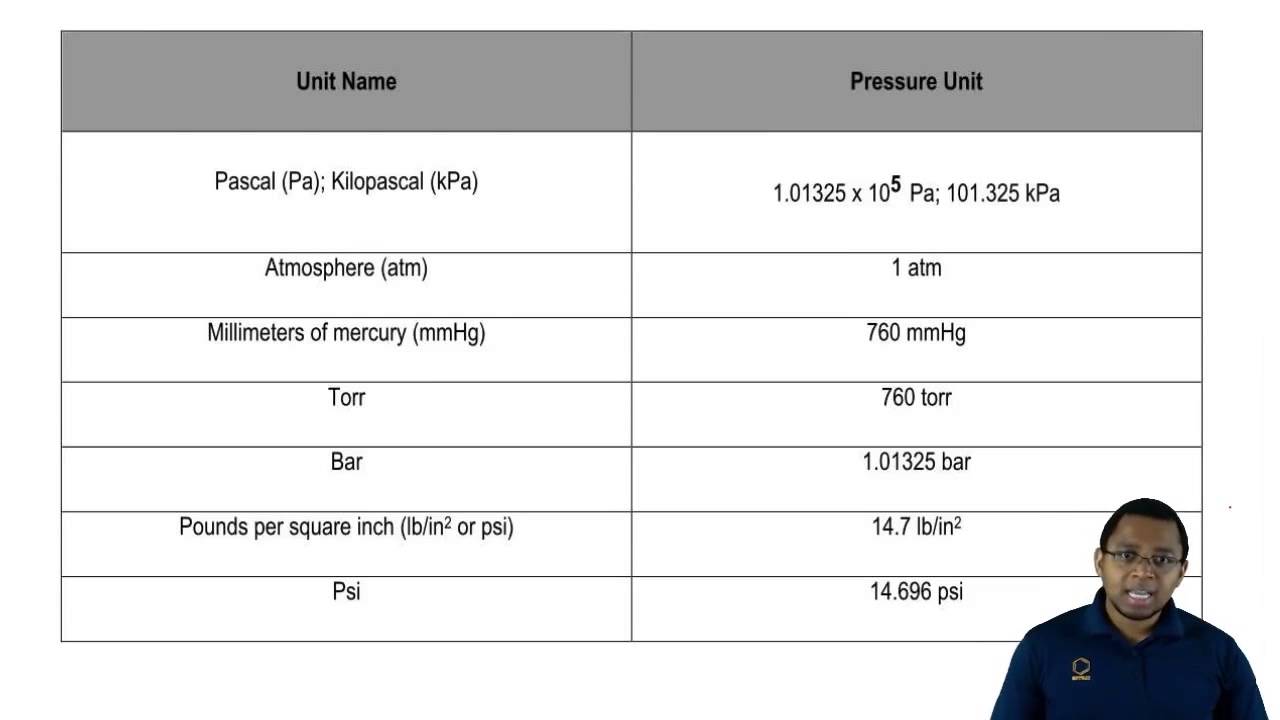
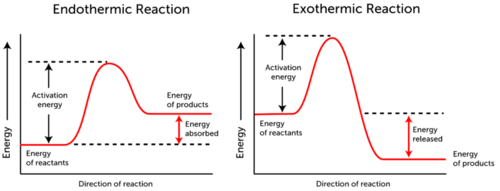
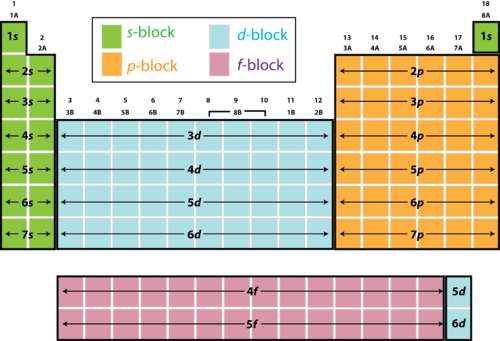
In our second lesson, we started to learn about electron configuration. We started by coloring our periodic table into the four sections, s,p,d,f; with this one class came up with Smart People Dropped First Semester to remember the different blocks. These blocks are used to create the identity along with the numbers. Here is what it looks like:(
https://dr282zn36sxxg.cloudfront.net/datastreams/f-d%3A0d99818a52b825e7cf4e51d8c68a3b0746467d8e519cd5fb7b6f914e%2BIMAGE_THUMB_POSTCARD%2BIMAGE_THUMB_POSTCARD.1
In the s block, there is 1 orbital, that can be filled with 2 electrons
The p block has 3 orbitals with 6 electrons
The d block has 5 orbitals with 10 electrons
The f block has 7 orbitals with 14 electrons
These are the maximum amounts of electrons that may fill up the orbitals that are present in each block. First they must go in with positive spin, and then fill in with negative spin once all the orbitals have filled up.
 |
| http://img.sparknotes.com/figures/0/083ee1e849c82204c3d7c342d336a448/fig1_5.gif |
We then learned about shortcuts when naming the elements with their configurations. For this, you can only use the noble gases, and they must be the most previous gas before the element you are trying to name, and you also cannot use a noble gas as its own shortcut.