https://dr282zn36sxxg.cloudfront.net/datastreams/f-d%3A0d99818a52b825e7cf4e51d8c68a3b0746467d8e519cd5fb7b6f914e%2BIMAGE_THUMB_POSTCARD%2BIMAGE_THUMB_POSTCARD.1
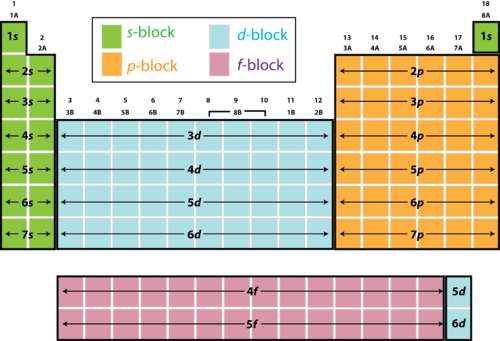
In the s block, there is 1 orbital, that can be filled with 2 electrons
The p block has 3 orbitals with 6 electrons
The d block has 5 orbitals with 10 electrons
The f block has 7 orbitals with 14 electrons
These are the maximum amounts of electrons that may fill up the orbitals that are present in each block. First they must go in with positive spin, and then fill in with negative spin once all the orbitals have filled up.
| http://img.sparknotes.com/figures/0/083ee1e849c82204c3d7c342d336a448/fig1_5.gif |
We then learned about shortcuts when naming the elements with their configurations. For this, you can only use the noble gases, and they must be the most previous gas before the element you are trying to name, and you also cannot use a noble gas as its own shortcut.
Holly, I found your post very educational. I liked how you explained each of the sublevels with their corresponding orbitals and electrons. In addition, I liked how you included the examples and shortcut of configurations. Your trick to remembering spdf was handy too! Thanks for the great post!
ReplyDelete