Sunday, December 13, 2015
Copper (II) Chloride Lab
Thursday and Friday in class we work on this new Copper (II) Chloride Lab. On Thursday, we measured the mass of the baby food jar first. Then my partner measured out 4g of Copper (II) Chloride and 50.0mL of water. While she was doing that, I was cleaning off our nail with steel wool so that the reaction could go faster. I took the mass of the nail and added it to the dissolved solution my partner had made.
On Friday, after it had rested for a complete 24 hours, there was some copper that had formed already. We took out the nail and washed it off into the jar. The bottom half of the nail that was in the solution looked to be about half as thick as it originally was. We then drained the liquid that was in the jar, and added 25mL of HCl to it. We then drained this out and added 25mL of distilled water and drained this also. At the end of this day, we were left with copper that is drying over this weekend.
Percent Yield
This lesson has been one of the simplest lessons we have learned this year in Chemistry. After calculating the theoretical yield and the actual yield, all there is to do is plug it into the formula. In the practice problems we were given, the problems themselves already gave us the yield, and all we had to do was use algebra to solve for either one or both of the yields. To find the theoretical yield sometimes all that must be done is the simple conversions we learned earlier.
This is how the problem is set up
These are some links that walk through the process:
http://study.com/academy/lesson/how-to-calculate-percent-yield-definition-formula-example.html
https://www.youtube.com/watch?v=Kk5kZO8ueWI
This is how the problem is set up
These are some links that walk through the process:
http://study.com/academy/lesson/how-to-calculate-percent-yield-definition-formula-example.html
https://www.youtube.com/watch?v=Kk5kZO8ueWI
Limiting Reagent and Excess Reagent
Monday in class we continued with the method that we had already learned, and applied it to solving for what are called the limiting reagent and the excess reagent. The limiting reagent whichever conversion ends up as the smaller number, and this number is the maximum amount of product that can be made. The limiting reagent is called this because the reaction will occur until this reactant runs out, and the other reactant in the reaction will be leftover in excess. The excess reagent is the larger number in these conversions, but its amount is not this number. To find the amount of excess reagent, you must take the amount of the limiting reagent and use the conversions to put it in the correct labeling for the excess. For example, if the limiting reagent was 10.5g NH3, then you would take the 10.5gNH3 and convert it to grams of Ca(OH)2, the excess reagent. Once this is converted, you will take that number and subtract it from the original amount, and finally you will know how much is left.
This link helped me the most, even though it has silly illustrations:https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/limiting-reagent-stoichiometry/a/limiting-reagents-and-percent-yield
Here are some other links that helped me with this lesson:
Website: https://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
Video: https://www.youtube.com/watch?v=qLUJdF_l8LA
These images show how some of the reactants form the products, but the red leftovers are the excess reagents we solve for.
This link helped me the most, even though it has silly illustrations:https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/limiting-reagent-stoichiometry/a/limiting-reagents-and-percent-yield
Here are some other links that helped me with this lesson:
Website: https://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
Video: https://www.youtube.com/watch?v=qLUJdF_l8LA
Thursday, December 10, 2015
Stoichiometry start
In the beginning of the Stoichiometry unit, we learned about the relationship between reactants and products in a chemical equation. We learned that by knowing just one value, we are able to calculate the values of the other reactants and products. We started by balancing a chemical equation then used the chart we were given, and personalized the problem while basing it on the chart. With this you are able to find what you want in either grams or moles, and it is very similar looking to simple conversions. Here is what the chart looks like that we used in class.:
Here are some links to help with the understanding of Stoichiometry:
Video: https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/stoichiometry-ideal/v/stoichiometry
Website: http://www.chemteam.info/Stoichiometry/Stoichiometry.html
Video: https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/stoichiometry-ideal/v/stoichiometry
Website: http://www.chemteam.info/Stoichiometry/Stoichiometry.html
Thursday, December 3, 2015
Oxidation Rules
This is a list of rules that we learned in class that we must use for classifying the oxidized and reduced substance, which are also the oxidizing agent and reducing agent.
1) Each atom in a pure element has an oxidation number of 0
2) Monatomic ions have an oxidation number equal to the charge on the ion
3) Fluorine always has an oxidation number of -1 in compounds with other elements
4) Cl, Br, and I always have an oxidation number of -1 in compounds, except when combined with oxygen or fluorine
5) The oxidation number of H is +1 and 0 is -2 in most compounds
There are exceptions:
in compounds with metals, H is -1
in peroxides, O has a charge of -1
6) The sum of the oxidation numbers for atoms in a neutral compound is 0
(no charge is shown at end)
In a polyatomic ion, the sum must be equal to the ion charge
Here are some references to go through the process of figuring them out:
Video: https://www.youtube.com/watch?v=81WdyqvLlVA
Website http://www.occc.edu/kmbailey/chem1115tutorials/oxidation_numbers.htm
1) Each atom in a pure element has an oxidation number of 0
2) Monatomic ions have an oxidation number equal to the charge on the ion
3) Fluorine always has an oxidation number of -1 in compounds with other elements
4) Cl, Br, and I always have an oxidation number of -1 in compounds, except when combined with oxygen or fluorine
5) The oxidation number of H is +1 and 0 is -2 in most compounds
There are exceptions:
in compounds with metals, H is -1
in peroxides, O has a charge of -1
6) The sum of the oxidation numbers for atoms in a neutral compound is 0
(no charge is shown at end)
In a polyatomic ion, the sum must be equal to the ion charge
| http://www.drcruzan.com/Images/Chemistry/OxidationNumbers/OxidationNumbersTable.png |
Here are some references to go through the process of figuring them out:
Video: https://www.youtube.com/watch?v=81WdyqvLlVA
Website http://www.occc.edu/kmbailey/chem1115tutorials/oxidation_numbers.htm
Metals Lab
Earlier this week we did a lab in class in which we tested the reactivity of several metals in different aqueous solutions. The lab was much more interesting to me than the others, because we could actually see the reactions happening through gas production, color changing and bubbling. We had to complete a chart on what we saw react and what we observed happening. From what we put down in our chart, we made our own reactivity series and this really helped me understand this concept. Here is a picture of one of the reactions that lasted longer, changed colors, and bubbled also:
Here's a link further explaining the reactivity series: http://www.cod.edu/people/faculty/jarman/richenda/1551_hons_materials/Activity%20series.htm
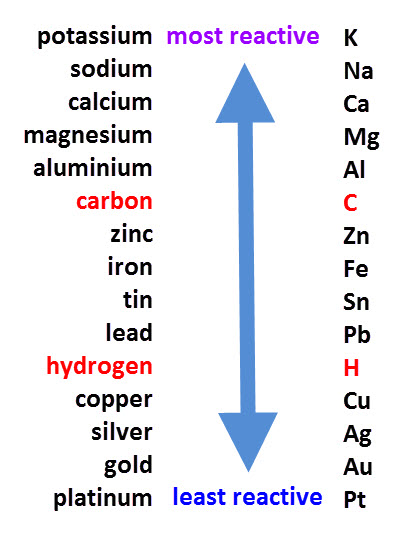
Now, this is what the standard reactivity series looks like. Those on top of the list are more reactive, and those toward the bottom are less. You use these comparisons in single replacement reactions.
Here's a link further explaining the reactivity series: http://www.cod.edu/people/faculty/jarman/richenda/1551_hons_materials/Activity%20series.htm
Now, this is what the standard reactivity series looks like. Those on top of the list are more reactive, and those toward the bottom are less. You use these comparisons in single replacement reactions.
 |
| Reactivity Series ADDED.jpg |
Subscribe to:
Comments (Atom)



