Our first lesson in this unit introduced us into the characteristics of gases and measuring pressures. We learned that ages expand spontaneously to fill their container, have no definite volume, are highly compressible, form homogeneous mixtures, and have molecules that are relatively far apart from one another.
Then, we went into converting into different units of pressure, where we had to memorize a similar chart:
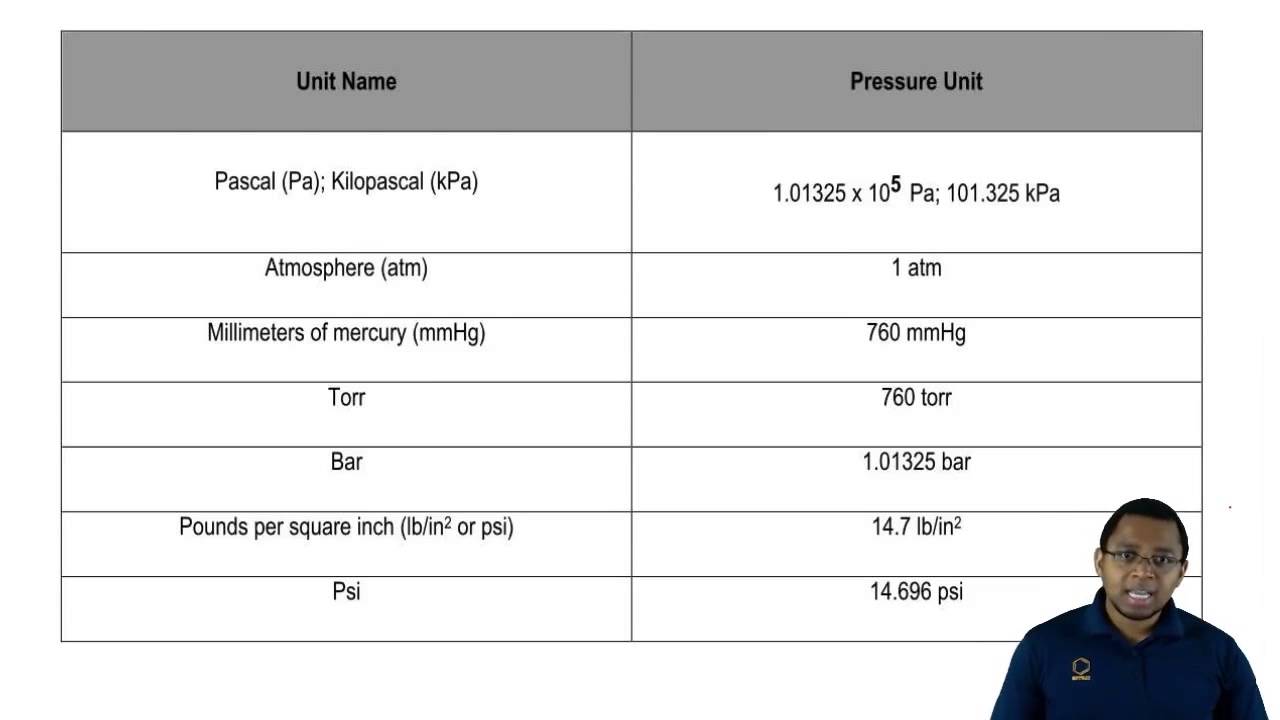 |
| https://i.ytimg.com/vi/mQn1Lat73AE/maxresdefault.jpg |
Then, we learned about Boyle's law, which come of the basis of:
 |
| http://patentimages.storage.googleapis.com/EP0023910B1/imgb0016.png |
This law states that relationship between pressure and volume is an inverse relationship, and holds true at constant temperature and moles. We then did some practice problems and looked at how the law works.
 |
| https://d2gne97vdumgn3.cloudfront.net/api/file/jMkrpsxQ5epbGnRIvowW |
Here are some links to help with this lesson and overall gasses: